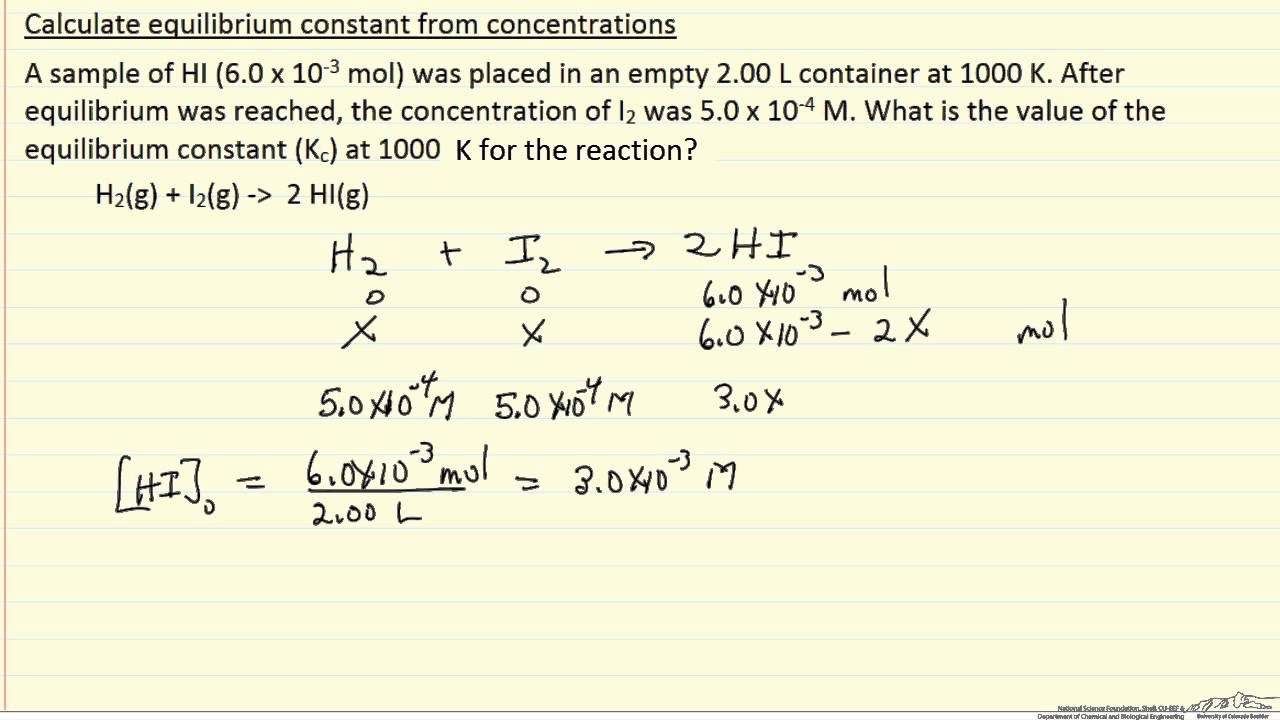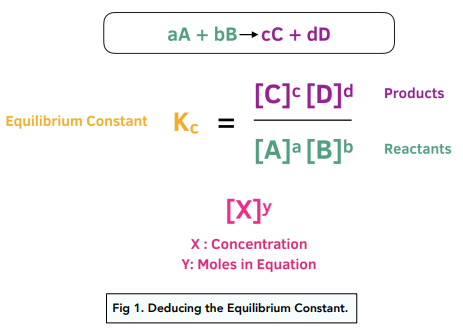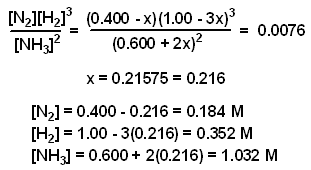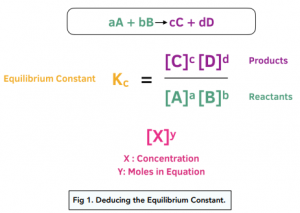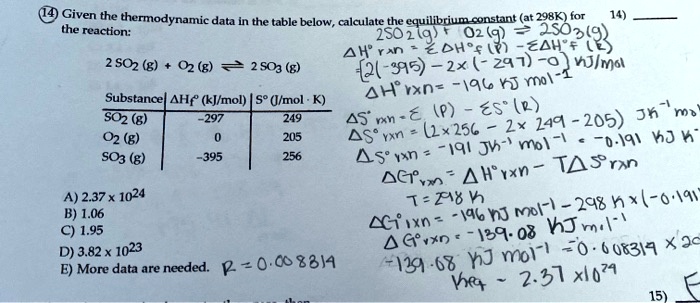
SOLVED: Given - the thermodynamic data in the table below, calculate the equilibrium constant (at 298K) for the reaction: 250 2 02 2502 9 3 Z4H" = 8 4h" in Dh"f SO2 (

Is the equilibrium constant calculation only applicable to homogeneous reactions? - Chemistry Stack Exchange
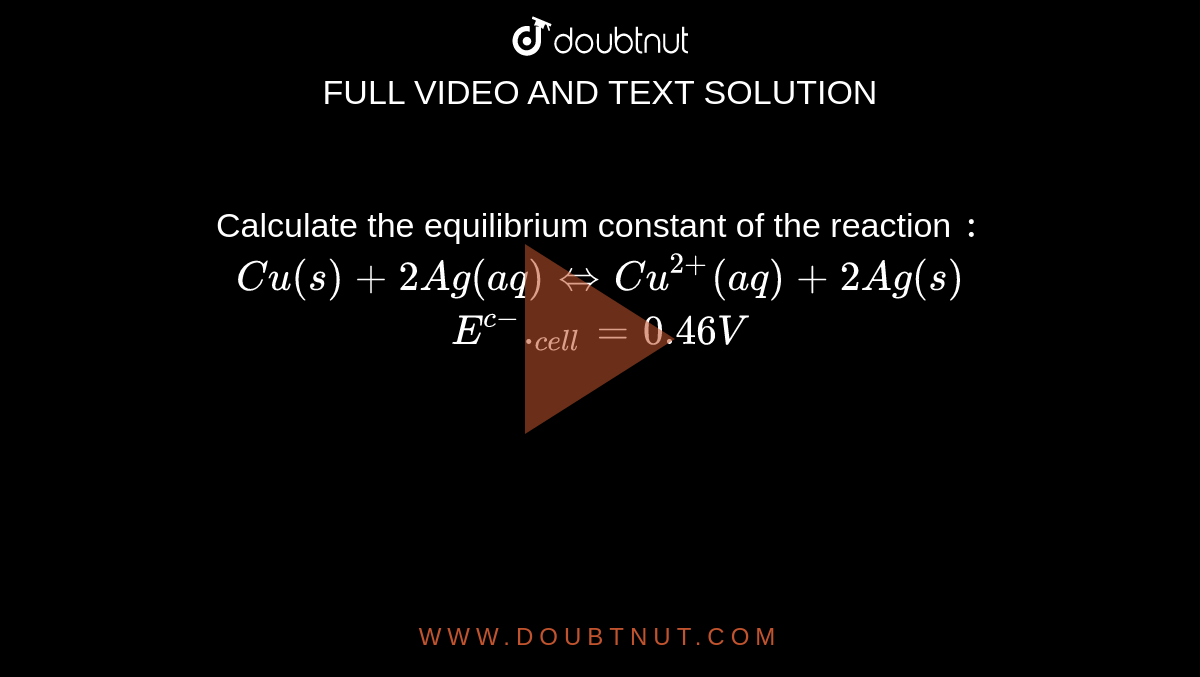
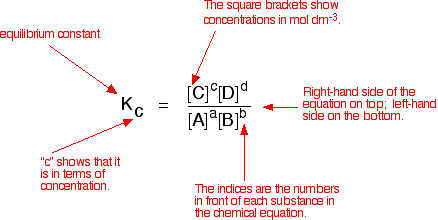
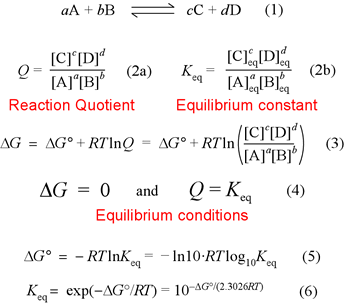
Calculate the equilibrium constant of the reaction : Cu(s)+2Ag(aq) hArrCu^(2+)(aq) +2Ag(s) E^(c-).(cell)=0.46V

Easy tricks to calculate equilibrium constant based problems/Chemical eq... | Simple tricks, Equilibrium, Problem

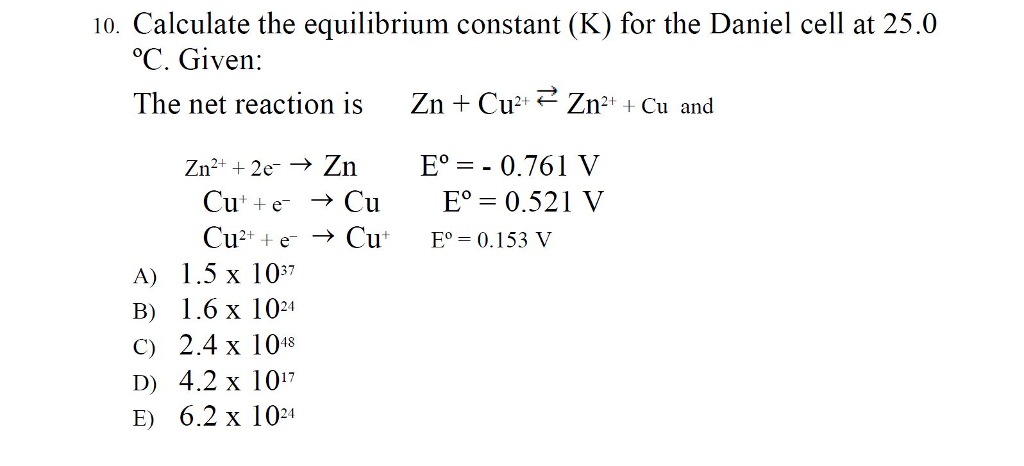
Calculate the equilibrium constant for the reaction at 298K. `Zn(s) +Cu^(2+)(aq) hArr Zn^(2+)(aq) +C - YouTube
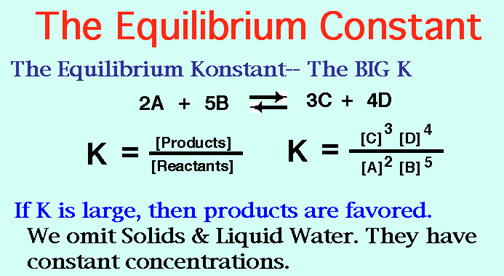
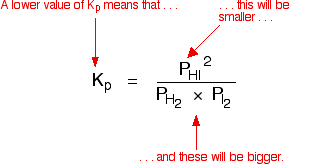
How does the value of an equilibrium constant relate to the relative quantities of reactants and products at equilibrium? | Socratic